The Element Argon
- Chemical Properties Of Argon
- Argon Atomic Mass
- Argon Atomic Radius
- Argon Atomic Symbol
- Argon Atomic Gold

Argon is a noble gas which composes about 1% of the atmosphere. It is used in incandescent light bulbs to permit the filament to be heated to a higher temperature, and therefore to produce a whiter light than would be practical in a vacuum. Argon (Ar) Energy Levels of Neutral Argon ( Ar I ) Configuration: Term: J: Level(cm-1): Ref. 3p 6: 1 S: 0: 0.000: VHU99: 3p 5 (2 P° 3 / 2)4s: 2 3 / 2°: 2.
[Click for Isotope Data]
Atomic Number: 18
Atomic Weight: 39.948
Melting Point: 83.80 K (-189.35°C or -308.83°F)
Boiling Point: 87.30 K (-185.85°C or -302.53°F)
Density: 0.0017837 grams per cubic centimeter
Phase at Room Temperature: Gas
Element Classification: Non-metal
Period Number: 3
Group Number: 18
Group Name: Noble Gas
What's in a name? From the Greek word for inactive, argos.
Say what? Argon is pronounced as AR-gon.
History and Uses:
Argon was discovered by Sir William Ramsay, a Scottish chemist, and Lord Rayleigh, an English chemist, in 1894. Argon makes up 0.93% of the earth's atmosphere, making it the third most abundant gas. Argon is obtained from the air as a byproduct of the production of oxygen and nitrogen.
Argon is frequently used when an inert atmosphere is needed. It is used to fill incandescent and fluorescent light bulbs to prevent oxygen from corroding the hot filament. Argon is also used to form inert atmospheres for arc welding, growing semiconductor crystals and processes that require shielding from other atmospheric gases.
Once thought to be completely inert, argon is known to form at least one compound. The synthesis of argon fluorohydride (HArF) was reported by Leonid Khriachtchev, Mika Pettersson, Nino Runeberg, Jan Lundell and Markku Räsänen in August of 2000. Stable only at very low temperatures, argon fluorohydride begins to decompose once it warms above -246°C (-411°F). Because of this limitation, argon fluorohydride has no uses outside of basic scientific research.
Estimated Crustal Abundance: 3.5 milligrams per kilogram
Estimated Oceanic Abundance: 4.5×10-1 milligrams per liter
Number of Stable Isotopes: 3 (View all isotope data)
Ionization Energy: 15.760 eV
Oxidation States: 0
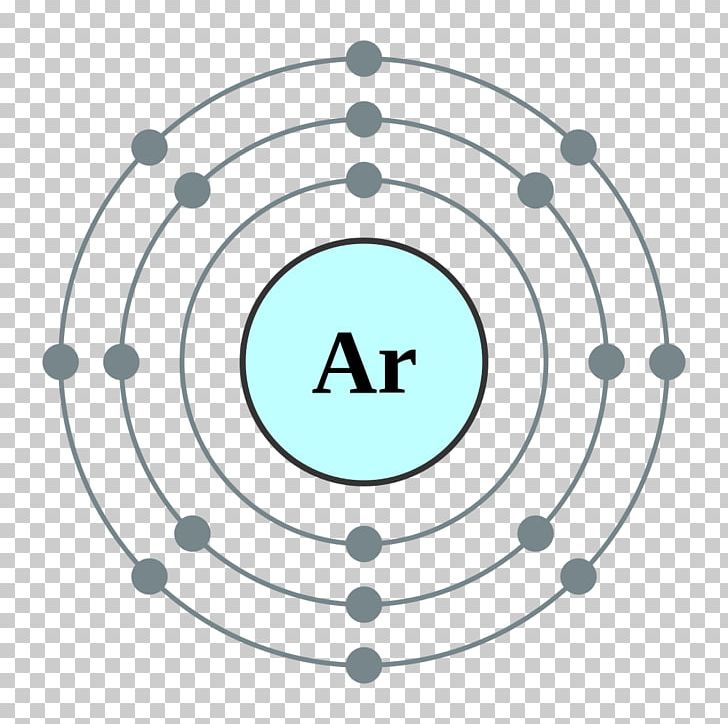
Electron Shell Configuration: | 1s2 |
2s2 2p6 | |
3s2 3p6 |
For questions about this page, please contact Steve Gagnon.
The Element Argon
[Click for Isotope Data]
Atomic Number: 18
Atomic Weight: 39.948
Melting Point: 83.80 K (-189.35°C or -308.83°F)
Boiling Point: 87.30 K (-185.85°C or -302.53°F)
Density: 0.0017837 grams per cubic centimeter
Phase at Room Temperature: Gas
Element Classification: Non-metal
Period Number: 3
Group Number: 18
Group Name: Noble Gas
What's in a name? From the Greek word for inactive, argos.
Say what? Argon is pronounced as AR-gon.
History and Uses:
Argon was discovered by Sir William Ramsay, a Scottish chemist, and Lord Rayleigh, an English chemist, in 1894. Argon makes up 0.93% of the earth's atmosphere, making it the third most abundant gas. Argon is obtained from the air as a byproduct of the production of oxygen and nitrogen.
Chemical Properties Of Argon

Argon is frequently used when an inert atmosphere is needed. It is used to fill incandescent and fluorescent light bulbs to prevent oxygen from corroding the hot filament. Argon is also used to form inert atmospheres for arc welding, growing semiconductor crystals and processes that require shielding from other atmospheric gases.
Once thought to be completely inert, argon is known to form at least one compound. The synthesis of argon fluorohydride (HArF) was reported by Leonid Khriachtchev, Mika Pettersson, Nino Runeberg, Jan Lundell and Markku Räsänen in August of 2000. Stable only at very low temperatures, argon fluorohydride begins to decompose once it warms above -246°C (-411°F). Because of this limitation, argon fluorohydride has no uses outside of basic scientific research.
Estimated Crustal Abundance: 3.5 milligrams per kilogram
Argon Atomic Mass
Estimated Oceanic Abundance: 4.5×10-1 milligrams per liter
Number of Stable Isotopes: 3 (View all isotope data)

Argon Atomic Radius
Ionization Energy: 15.760 eV
Argon Atomic Symbol
Oxidation States: 0
Argon Atomic Gold
Electron Shell Configuration: | 1s2 |
2s2 2p6 | |
3s2 3p6 |

For questions about this page, please contact Steve Gagnon.




