Molar mass serves as a bridge between the mass of a material and the number of moles since it is not possible to measure the number of moles directly. Key Terms molar mass: The mass of a given substance (chemical element or chemical compound in g) divided by its amount of substance (mol).
- Medical Definition of mass 1: the property of a body that is a measure of its inertia, that is commonly taken as a measure of the amount of material it contains, that causes it to have weight in a gravitational field, and that along with length and time constitutes one of the fundamental quantities on which all physical measurements are based.
- An object has mass (say 100 kg). This makes it heavy enough to show a weight of '100 kg'.
- Mass fraction can also be expressed, with a denominator of 100, as percentage by mass (in commercial contexts often called percentage by weight, abbreviated wt%; see mass versus weight). It is one way of expressing the composition of a mixture in a dimensionless size; mole fraction (percentage by moles, mol%) and volume fraction ( percentage.
Mass vs. weight - the Gravity Force
Mass and Weight are two often misused and misunderstood terms in mechanics and fluid mechanics.
The fundamental relation between mass and weight is defined by Newton's Second Law. Newton's Second Law can be expressed as
F = m a (1)
where
F = force (N, lbf)

m = mass (kg, slugs)
a = acceleration (m/s2, ft/s2)
Mass
Mass is a measure of the amount of material in an object, being directly related to the number and type of atoms present in the object. Mass does not change with a body's position, movement or alteration of its shape, unless material is added or removed.
- an object with mass 1 kg on earth would have the same mass of 1 kg on the moon
Mass is a fundamental property of an object, a numerical measure of its inertia and a fundamental measure of the amount of matter in the object.
- mass electron 9.1095 10-31 kg
- mass proton 1.67265 10-27 kg
- mass neutron 1.67495 10-27 kg
Weight
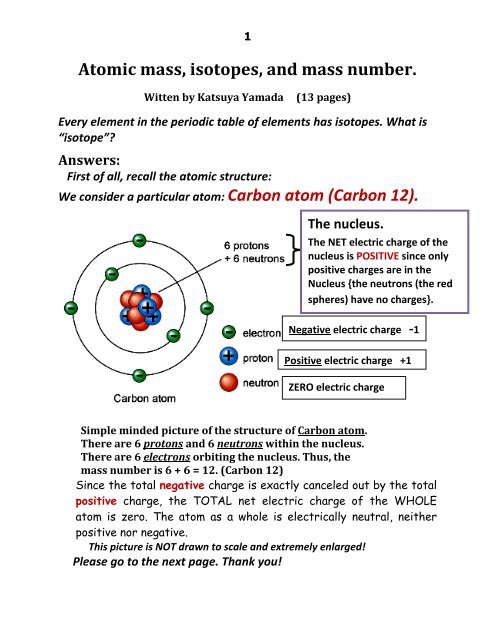
Weight is the gravitational force acting on a body mass. The generic expression of Newton's Second Law(1) can be transformed to express weight as a force by replacing the acceleration - a - with the acceleration of gravity - g - as
Fg = m ag (2)
where
Fg = gravitational force - or weight (N, lbf)
m = mass (kg, slugs (lbm))
ag = acceleration of gravity on earth (9.81 m/s2, 32.17405 ft/s2)
Example - The Weight of a Body on Earth vs. Moon
The acceleration of gravity on the moon is approximately 1/6 of the acceleration of gravity on the earth. The weight of a body with mass 1 kg on the earth can be calculated as
Fg_earth = (1 kg) (9.81 m/s2)
= 9.81 N
The weight of the same body on the moon can be calculated as
Fg_moon = (1 kg) ((9.81 m/s2) / 6)
= 1.64 N
The handling of mass and weight depends on the systems of units used. The most common unit systems are
- the International System - SI
- the British Gravitational System - BG
- the English Engineering System - EE
One newton is
- ≈ the weight of one hundred grams - 101.972 gf (gF) or 0.101972 kgf (kgF or kilopond - kp (pondus is latin for weight))
- ≈ halfway between one-fifth and one-fourth of a pound - 0.224809 lb or 3.59694 oz
The International System - SI
In the SI system the mass unit is the kg and since the weight is a force - the weight unit is the Newton (N). Equation (2) for a body with 1 kg mass can be expressed as:
Fg = (1 kg) (9.807 m/s2)
= 9.807 (N)
where
9.807 m/s2 = standard gravity close to earth in the SI system
As a result:
- a 9.807 N force acting on a body with 1 kg mass will give the body an acceleration of 9.807 m/s2
- a body with mass of 1 kg weights 9.807 N
- More about the SI System - A tutorial introduction to the SI-system.
The Imperial British Gravitational System - BG
The British Gravitational System (Imperial System) of units is used by engineers in the English-speaking world with the same relation to the foot - pound - second system as the meter - kilogram - force second system (SI) has to the meter - kilogram - second system. For engineers who deals with forces, instead of masses, it's convenient to use a system that has as its base units length, time, and force, instead of length, time and mass.
The three base units in the Imperial system are foot, second and pound-force.
In the BG system the mass unit is the slug and is defined from the Newton's Second Law (1). The unit of mass, the slug, is derived from the pound-force by defining it as the mass that will accelerate with 1 foot per second per second when a 1 pound-force acts upon it:
1 lbf = (1 slug) (1 ft/s2)
Mass Number Is Determined By
In other words, 1 lbf (pound-force) acting on 1 slug of mass will give the mass an acceleration of 1 ft/s2.
The weight (force) of the mass can be calculated from equation (2) in BG units as
Fg (lbf) = m (slugs) ag (ft/s2)
With standard gravity - ag = 32.17405 ft/s2 - the weight (force) of 1 slug mass can be calculated as
Fg = (1 slug) (32.17405 ft/s2)
= 32.17405 lbf
The English Engineering System - EE
In the English Engineering system of units the primary dimensions are are force, mass, length, time and temperature. The units for force and mass are defined independently
- the basic unit of mass is pound-mass (lbm)
- the unit of force is the pound (lb) alternatively pound-force (lbf).
In the EE system 1 lbf of force will give a mass of 1 lbm a standard acceleration of 32.17405 ft/s2.
Since the EE system operates with these units of force and mass, the Newton's Second Law can be modified to
F = m a / gc (3)
where
gc = a proportionality constant
or transformed to weight (force)
Fg = m ag / gc (4)
The proportionality constant gc makes it possible to define suitable units for force and mass. We can transform (4) to
1 lbf = (1 lbm) (32.174 ft/s2) / gc
or
gc = (1 lbm) (32.174 ft/s2) / (1 lbf)
Since 1 lbf gives a mass of 1 lbm an acceleration of 32.17405 ft/s2 and a mass of 1 slug an acceleration of 1 ft/s2, then
1 slug = 32.17405 lbm
Example - Weight versus Mass
The mass of a car is 1644 kg. The weight can be calculated:
Fg = (1644 kg) (9.807 m/s2)
= 16122.7 N
= 16.1 kN
- there is a force (weight) of 16.1 kN between the car and the earth.
- 1 kg gravitation force = 9.81 N = 2.20462 lbf
Weight Converter
weight
(kgf)
(N)
(lbf)
Kg to lb Converter
Related Topics
- Basics - The SI-system, unit converters, physical constants, drawing scales and more
- Fluid Mechanics - The study of fluids - liquids and gases. Involves velocity, pressure, density and temperature as functions of space and time
- Mechanics - Forces, acceleration, displacement, vectors, motion, momentum, energy of objects and more
- Statics - Loads - force and torque, beams and columns
Related Documents
- Acceleration - Change in velocity and time used
- Acceleration of Gravity and Newton's Second Law - Acceleration of gravity and Newton's Second Law - SI and Imperial units
- Acceleration Units Converter - Convert between units of acceleration
- Acetone - Density and Specific Weight - Online calculator, figures and tables showing density and specific weight of acetone at temperatures ranging from -95 to 275 °C (-138 to 530 °F) at atmospheric and higher pressure - Imperial and SI Units
- Ammonia - Density at Varying Temperature and Pressure - Online calculator, figures and tables showing density and specific weight of ammonia at temperatures ranging -50 to 425 °C (-50 to 800 °F) at atmospheric and higher pressure - Imperial and SI Units
- Apothecaries' Weight System - Apothecaries' fluid and weight system - ounce, drachms, grains, scruples ..
- Argon - Density and Specific Weight - Online calculator, figures and tables showing density and specific weight of argon, Ar, at varying temperature and pressure - Imperial and SI Units
- Benzene - Density and Specific Weight - Online calculator, figures and table showing density and specific weight of benzene, C6H6, at temperatures ranging from 5 to 325 °C (42 to 620 °F) at atmospheric and higher pressure - Imperial and SI Units
- Bollard Force - Rope friction around a pole - load and effort force in rope around a bollard
- Butane - Density and Specific Weight - Online calculators, figures and tables showing density and specific weight of liquid and gaseous butane, C4H10, at varying temperarure and pressure, SI and Imperial units
- Center Mass - Calculate position of center mass
- Center of Gravity - Bodies and their center of gravity
- Convert Gallons of Water to Pounds - and vice versa
- Density of Selected Solids - Density of selected solids
- Density, Specific Weight and Specific Gravity - An introduction to density, specific gravity and specific weight - formulas with examples
- Drying Force of Air - The drying force of air can be expressed with moisture holding capacity of air and the evaporation capacity from water surface to air
- Ethane - Density and Specific Weight - Online calculator, figures and tables showing density and specific weight of ethane, C2H6, at varying temperature and pressure - Imperial and SI Units
- Ethylene - Density and Specific Weight - Online calculator, figures and tables showing density and specific weight of ethylene, C2H4, at varying temperature and pressure - Imperial and SI Units
- Force - Newton's third law - mass and acceleration
- Force acting on Body Moving in Horizontal Plane - The force acting on a body moved in the horizontal plane
- Forces Acting on Body Moving on an Inclined Plane - Required force to move a body up an inclined plane
- Gases - Densities - Densities and molecular weights of some common gases - acetylene, air, methane, nitrogen, oxygen and others ..
- Gears - Gears and effort force
- Helium - Density and Specific Weight - Online calculator, figures and tables showing density and specific weight of helium, He, at varying temperature and pressure - Imperial and SI Units
- Hot Air Balloon - Lifting Force - Calculate potential lifting force in hot air volume
- Levers - Use levers to magnify forces
- Lifting Wheels - Lifting wheels - loads and effort force
- Liquids - Densities - Densities of common liquids like acetone, beer, oil, water and more
- Mass Moment of Inertia - Mass Moment of Inertia (Moment of Inertia) depends on the mass of the object, its shape and its relative point of rotation - Radius of Gyration
- Methane - Density and Specific Weight - Online calculator, figures and tables showing density and specific weight of methane, CH4, at temperatures ranging from -160 to 725 °C (-260 to 1300 °F) at atmospheric and higher pressure - Imperial and SI Units
- Methanol - Density and Specific Weight - Online calculator, figures and tables showing density and specific weight of methanol,CH3OH, at varying temperature and pressure - Imperial and SI Units
- Nitrogen - Density and Specific Weight - Online calculator, figures and tables showing density and specific weight of nitrogen, N2, at temperatures ranging from -175 to 1325 °C (-280 to 2400 °F) at atmospheric and higher pressure - Imperial and SI Units
- Ounces and Pounds converted to Grams - Converting ounces (ozs.) and pounds (lbs.) to grams (g)
- Oxygen - Density and Specific Weight - Online calculator, figures and tables showing density and specific weight of oxygen, O2, at varying temperature and pressure - Imperial and SI Units
- Propane - Density and Specific Weight - Online calculator, figures and tables showing density and specific weight of propane, C3H8, at temperatures ranging from -187 to 725 °C (-305 to 1300 °F) at atmospheric and higher pressure - Imperial and SI Units
- Pulleys - Pulleys, blocks and tackles
- SI System - An introduction to the SI-system
- Support Reactions - Equilibrium - Static equilibrium is achieved when the resultant force and resultant moment equals to zero
- Threaded Rods - Proof Loads in Metric Units - Proof load capacities of metric threaded steel rods
- Toluene - Density and Specific Weight - Linear temperature expansion coefficients for aluminum, copper, glass, iron and other common materials
- Troy Weight - Weight of gold and silver
- Universal Gravitational Law - The gravitational attraction between two objects depends on the mass of the objects and the distance between them
- Water - Density, Specific Weight and Thermal Expansion Coefficient - Definitions, online calculator, figures and tables giving Density, Specific Weight and Thermal Expansion Coefficient of liquid water at temperatures ranging from 0 to 360 °C and 32 to 680°F - in Imperial and SI Units
Tag Search
- en: mass weight newton force kg lb slugs
- es: masa de fuerza newton peso kg babosas libras
- de: Massengewicht Newton Kraft kg lb Schnecken
Q: Aren't 'weight' and 'mass' the same?
A: Not really.
An object has mass (say 100 kg). This makes it heavy enough to show a weight of '100 kg'. |
But the scales are only showing a guess of the mass above them!
Gravity causes Weight
An object's weight is how hard gravity is pulling on it.
We think the weight is the same everywhere ... because we all live on the surface of the planet Earth!
But in orbit it would not push on the scales at all.
The scales would show 0 ...
... but the mass is still 100 kg !
An object's mass doesn't change (unless you remove some!), but its weight can change.
On the Moon the scales would wrongly show 16.6
for a mass of 100 kg
Because the pull of gravity on the Moon
is much less than on Earth
So Why Do People Say Weight instead of Mass?
People often use 'weight' to mean 'mass', and vice versa, because Gravity is almost the same everywhere on Earth and we don't notice a difference.
Mass Number Is The Number Of Protons
But remember .. they do not mean the same thing,
and they can have different measurements.
Weight is a Force
So ... if weight and mass are different, why are they both in kilograms?
Well, weight should not really be in kilograms!
Mass Number Isotope
I have used 'kilogram' so far because that is what you see on a pair of scales, but it is technically wrong to talk about weight in kilograms ...
... weight is a force ...
... which is measured in Newtons
Newtons
The correct unit for force is the Newton (=1 kg·m/s2) which is abbreviated N.
| On the Earth's surface gravity makes a 1 kilogram mass exert about 9.8 Newtons of force |
So a 100 kg mass really weighs about 980 Newtons on Earth.
Why Do Scales Show Kilograms?
Scales show Kilograms because that is what people understand best ...
... but it is really just an estimate of the mass above them.
Scales should really show Newtons, but that might confuse people!
Question: how many Newtons should the scales show when you stand on them (hint: multiply kg by 9.8)?
- So the scales show an estimate of your mass based on the force your body exerts on it.
- And to find out how much force your body is exerting on the scales, multiply by 9.8 (to convert kg into Newtons).
Apparent Weight
But scales can be fooled ... because they measure a 'downwards force' and don't know if it is gravity or some other force!
Just jump up and down (gently!) on your scales at home to see your apparent weight change, while your mass stays the same.
So your mass is the same, and your weight is the same (because the force of gravity hasn't changed), but your 'apparent' weight changes. Read more at Apparent Weight
Conclusion
- Mass is a measure of how much matter something contains
- Weight is a measure of how strongly gravity pulls
- Apparent Weight is a measure of downwards force
- Force is measured in Newtons, not kilograms
- When scales show 'kg' it is just an estimate of the mass above them




